[最新] fractional yield formula chemical engineering 162825-Fractional yield formula chemical engineering
The theoretical molar yield is mol (the molar amount of the limiting compound, acetic acid) The molar yield of the product is calculated from its weight (132 g ÷ g/mol = 15 mol) The % yield is calculated from the actual molar yield and the theoretical molar yield (15 mol ÷ mol × 100% = 75%)In a chemical reaction, one or more reactants are transformed into products reactants → products The purpose of a chemical equation is to express this relation in terms of the formulas of the actual reactants and products that define a particular chemical change For example, the reaction of mercury with oxygen to produce mercuric oxide would be expressed by the equationAsCAvaries throughout the reactor, '?' will also vary with position in thereactorOverall fraction yield (?) is the mean of the instantaneous fractional yields at all points within the reactorBoth the yields depend on the typeof flow with in thereactor?
Chapter 6 Summary Notes
Fractional yield formula chemical engineering
Fractional yield formula chemical engineering-Acid HCV 15 S with a Single Fractional Distillation Column Muhammad Yusuf Ritonga1 1Chemical Engineering Department, Faculty of Engineering, University of Sumatera Utara, Jl Almamater Kampus USU, Medan 155, Indonesia Email yusufrit@gmailcom Abstract In 05 stearic acid V 15 S is manufactured forNiques used in the field of chemical engineering as well as biological, petroleum, and environmental engineering Although the range of subjects deemed to be in the province of chemical engineering has broadened over the last twenty years, the basic principles of this field of study remain the same


The Pillars Curriculum For Chemical Engineering
This is a Fluid Mechanics Formula Race wherein we will learn "Mechanical Operation" for the GATE Exam Chemical with Sumit Sir Use Code "PRAX100" to get 10%Overview of Fractional Distillation – As we know that fractional distillation is very important for chemical engineers, process Engineers and project engineers In this article we will study "what is fractional distillation " what is distillation , lab level distillation set up , large industrial level fractional distillation , small scale fractional distillation ,and medium scaleNiques used in the field of chemical engineering as well as biological, petroleum, and environmental engineering Although the range of subjects deemed to be in the province of chemical engineering has broadened over the last twenty years, the basic principles of this field of study remain the same
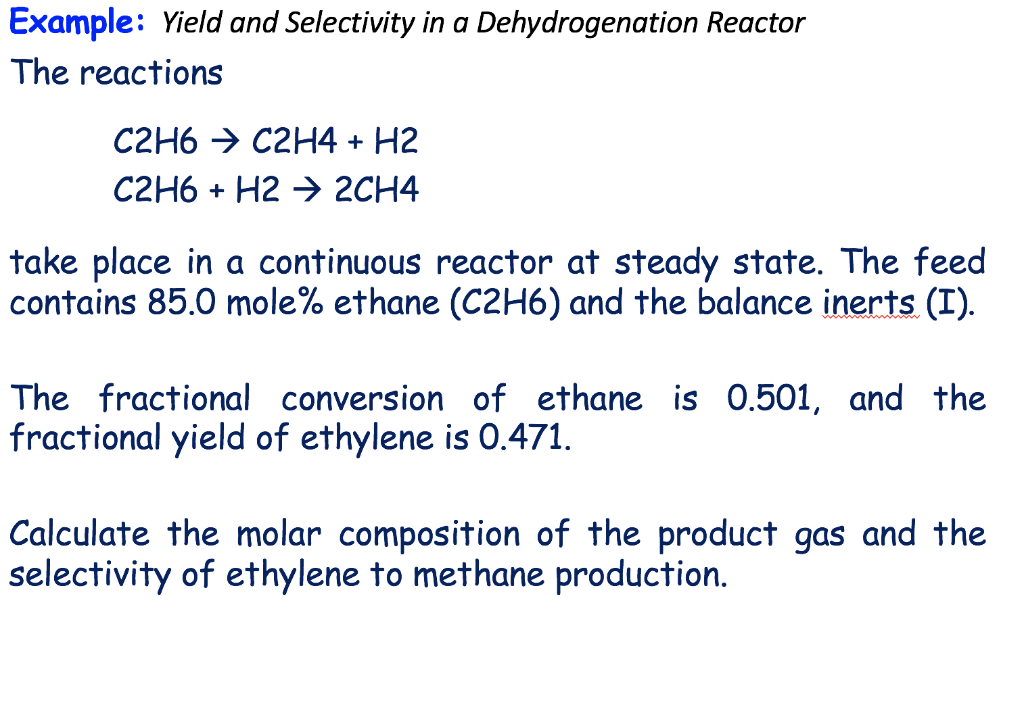
Prof Manolito E Bambase Jr Department of Chemical Engineering University of the Philippines Los Baños SLIDE 3 Molecular and Elemental Balances For steadystate reactive processes, Input Generation = Output Consumption The generation and consumption terms in the molecular balance equation is usually obtained from chemical stoichiometryRaoult' s Law states that the vapor pressure of the solution is equal to mole fraction of the solvent multiplied by the vapor pressure of the pure solvent In general, this equation states that theWith many chemical and biochemical processes, we want to know the ratio of a reactant that is converted to a particular product This leads to the concept of yield We know that the fractional conversion has a value between 0 and 1, which is easy to visualize and conceptually accepted For the yield, we want to retain the same range
Distillation use Raoult's law or Dalton'so law of partial pressure or total pressure for separation of chemical components As per Raoult's low " partial pressure of components is equal to vapor pressure multiply by its mole fraction"X 4 = 6 In an absorption tower (or absorber), a gas is contacted with a liquid such that one or more components in the gas is transferred in the liquidChemical Engineering Design Towler Richard Adio Download PDF Download Full PDF Package This paper A short summary of this paper 29 Full PDFs related to this paper READ PAPER Chemical Engineering Design Towler Download Chemical Engineering Design Towler



The Effects Of Mixing Reaction Rates And Stoichiometry On Yield For Mixing Sensitive Reactions Part I Model Development


Www Diva Portal Org Smash Get Diva2 Attachment01 Pdf
In chemistry, yield (also called chemical yield or reaction yield) is the amount of product resulting from a chemical reaction The absolute yield gives the weight in grams, and the molar yield gives the number of moles The fractional yield, relative yield, or percentage yield shows how completely a synthetic procedure worked It is calculated by dividing the amount of product by the1 Department of Chemical and Materials Engineering, Faculty of Engineering, King Abdulaziz University, PO Box 804, Jeddah 215, Saudi Arabia 2 Department of Mathematics and Computer Sciences, Faculty of Art and Sciences, Cankaya University, Ankara, Turkey 3 Institute of Space Sciences, PO Box MG23, Magurele Bucharest, Romania 4 Department of Mathematics, Anand InternationalAn example is steam distillation of flowers to yield volatile oil and a waterbased distillate Fractional Distillation Fractional distillation is employed when the boiling points of the components of a mix are near to each other, as determined using Raoult's law


Http Www Velhightech Com Documents Bt6604 Cre Pdf


Q Tbn And9gctogwfl Rwrzxdwdr7jjndqx A3cagfs2a5kyfhyqeoh9p91tda Usqp Cau
Fractional Distillation Process Fractional Distillation ProcessThis type of distillation process is usually used industrially to get large amounts of products and also to get products in differentdifferent compositions called fractionsPrinciple of Fractional Distillation Process If we boil a multicomponent mixture then those components which have relatively lower boiling points willThis is a Fluid Mechanics Formula Race wherein we will learn "Concepts of Fluid Mechanics" for the GATE Exam Chemical with Sumit Sir Use Code "PRAX100" to gFor the given parallel reaction, the instantaneous fraction yield of 'S' with respect to 'A' is (S/A) = dCS / (dCA dCS dCT) = 2 CA / (1 2 CA C) = 2 CA / (1 CA) 2 Given, XA = 05 or CA = CAo (1 – XA) = 2 (1 – 05) = 1 gmol/lit


Http Faculty Poly Edu Rlevicky Handout3 Pdf


Chapter 3 Material Balance Part Ii Ppt Video Online Download
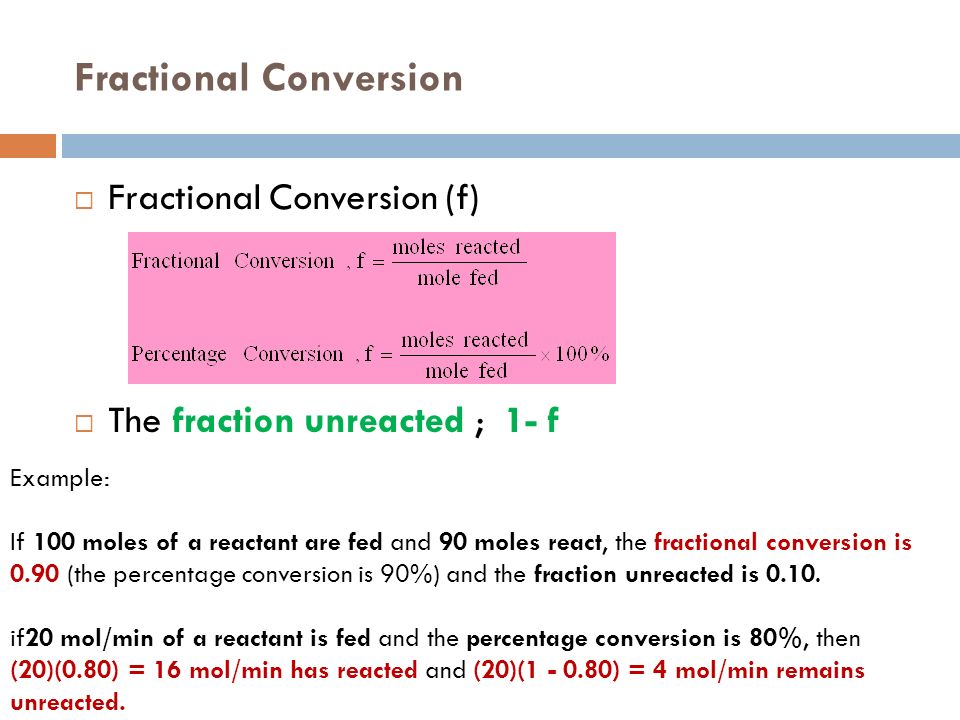
Conversion The fraction (or percentage) of the reactants that actually reacted during the reaction amount ofreactant converted %Conversion = 100 amount of reactant supplied Degree of Completion The fraction (or percentage) of the limiting reactant converted into products amount oflim reactant converted DegreeofCompletion = 100 amount of lim reactant supplied For example, in a chemical transformation composed of three reactions and with partial yields each of 25%, 50%, and 75%, respectively, to calculate the total yield the above equation is applied (expressing the partial yields to decimals), resulting the following expression (29) Total yield (%) = (025 × 050 × 075) × 100 = 94 %Fractional yield versus concentration when α 1 > α 2 CSTR PFR φ CA φ CA Af C A0 C Af C A0 C ΦCSTRΔCA ΦPFRΔCA Figure 10 Comparison of overall fractional yield for a CSTR and a PFR when α 1 > α 2 PFR is preferred because ΦPFR CSTR>Φ, therefore the yield of D per mol A consumed is higher If α12


Chapter 3 Material Balance Part Ii Ppt Video Online Download


Faculty Kfupm Edu Sa Che Aljuhani New Folder Material balance Pdf
A0 = entering mole fraction of A P t i ttl (kP) 0 0 0 0 0 0 0 R T F Cv A A A ⋅ = ⋅ 0 = entering total pressure (kPa) C A0 = entering conc'n (mol/dm 3) R = 14 kPa dm3 / mol K T = T(K) CSTR (Design Equation) D a d C a c B a b For a rxn A → F F A A A r V − − = 0 Substitute for F A A A A A F F F X V F F F X 0 ( 0 0) 0 − − ⋅ = = − ⋅ A exit A A r F X V r 0 − ⋅ = −So the catalyst which gives better C5 Reformate yield and lower coke formation is having better selectivity Fractional Conversion is defined as say XA = (NA0 NA) / NA0 where XA is the conversion rate of a reactant A, NA0 is the initial concentration of A & NA is the final concentration of A after the reaction takes placeCHEMICAL ENGINEERING (continued) 102 Common Names and Molecular Formulas of Some Industrial (Inorganic and Organic) Chemicals Common Name Chemical Name Molecular Formula Muriatic acid Hydrochloric acid HCl Cumene Isopropyl benzene C6H5CH(CH3)2 Styrene Vinyl benzene C6H5CH = CH2 — Hypochlorite ion OCl–1 — Chlorite ion ClO2 –1


Http Faculty Poly Edu Rlevicky Handout3 Pdf


Q Tbn And9gcqbx4lhvwffwq2jalu0eardaoc5r99ldcrwpwdghn9sl8ldwbrs Usqp Cau
Fractional conversion of a reactant A is defined as fractional reactant converted into product at any time It is given by the equation, XA = (NAO – NA) / NAO Where 'NAO' is the initial no of moles of reactant 'A' at t = 0 'NA' is the remaining no of moles of reactant at any time 't' in the reaction 32XO2 = ( x 103 mol / (45 x 103 mol 10 x 103 mol) XO2 = 10 x 103 / 55 x 103 XO2 = 018 Example 2 Determine the mole fraction of CH3OH and H2O in a solution prepared by dissolving 55 g of alcohol in 40 g of H2O M of H2O is 18 and M of CH3OH is 32So the catalyst which gives better C5 Reformate yield and lower coke formation is having better selectivity Fractional Conversion is defined as say XA = (NA0 NA) / NA0 where XA is the conversion rate of a reactant A, NA0 is the initial concentration of A & NA is the final concentration of A after the reaction takes place


Solved Example Yield And Selectivity In A Dehydrogenatio Chegg Com


Www Researchgate Net Profile Prem Baboo Post What Experiments Can Be Found Limiting Reagent Of A Chemical Reaction Between Mass Transfer And Kinetics Attachment 59d6487fba328f As 3a Download Fundchemreaxengch1 Pdf
Modern chemical engineering combines knowledge of chemistry and molecular interactions with the discipline of engineering to address problems at both the small scale and the large scale Chemical engineers invent new processes, improve existing ones and design and operate plants and equipment to transform raw feed stocks142a Percentage yield of the product of a reaction Even though no atoms are gained or lost in a chemical reaction (law of conservation of mass), unfortunately it is not always possible to obtain the calculated amount of a product (ie 100% yield) because the reaction may not go to completion because it may be reversible or some of the product may be lost when it is separated from theFor example, in a chemical transformation composed of three reactions and with partial yields each of 25%, 50%, and 75%, respectively, to calculate the total yield the above equation is applied (expressing the partial yields to decimals), resulting the following expression (29) Total yield (%) = (025 × 050 × 075) × 100 = 94 %


Advanced Chemical Reaction Engineering Part 1 10 Apr 16


2
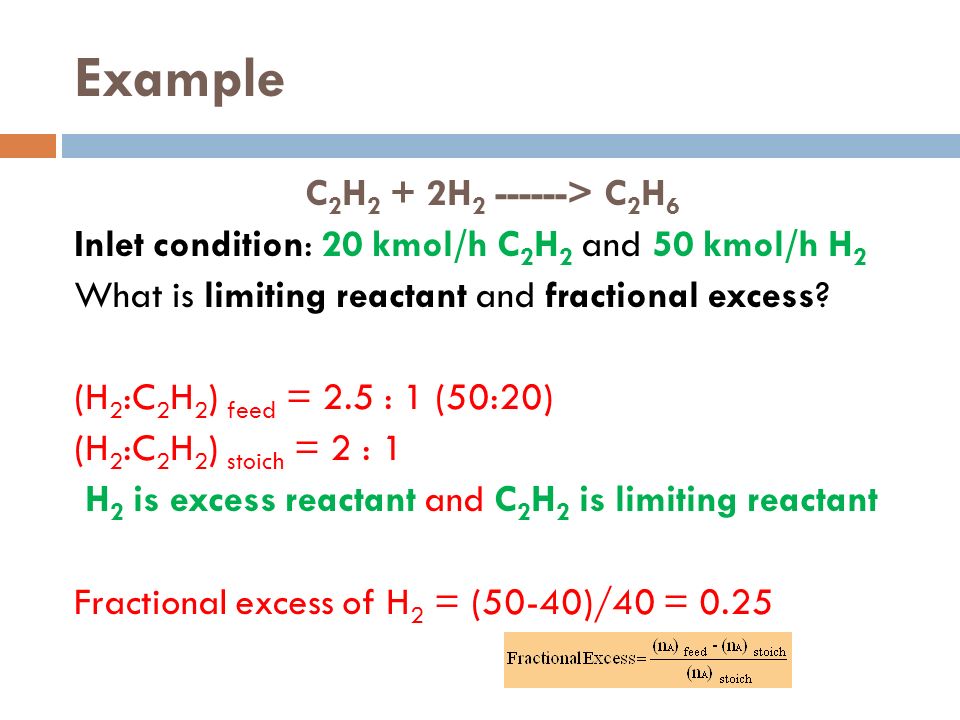
Percent Yield Formula The percent yield formula is taken into account to find out the % yield value The Percentage yield is determined by divide the actual yield with the theoretical yield and multiplied by 100% The percent yield equation is given below percent yield = (actual yield/theoretical yield) x 100%The reactant that would run out before the reaction proceeded to completion is called the limiting reactant, and the other reactants are termed excess reactants The fractional excess of the reactant is the ratio of the excess to the stoichiometric requirements Fractional excess of A = (moles A)feed – (moles A)stoich/(moles A)stoich The extent ofDetermine the fractional yield of fresh water from the process (kg H 2O recovered/kg H 2O in process feed) and the fraction of salt in the solution leaving the fourth evaporator Ans Y W = 031 ;



Chapter 11 Combustion Updated 5 31 10



The Fertiliser Industry The Chemical Industry Siyavula
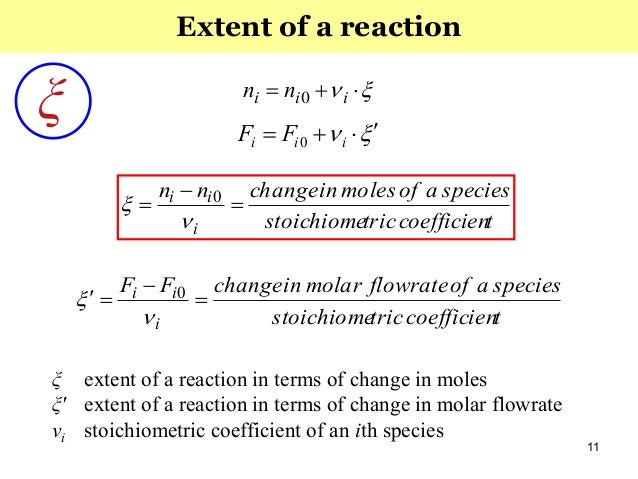
253 – Yield (Fractional Yield) ¶ Yield is defined as the moles of product formed divided by the ideal number of moles formed Y i e l d = n ˙ f o r m e d n ˙ i d e a l The ideal number of moles formed is assuming that the limiting reactant reacts fully and that there are no side reactionsSo the catalyst which gives better C5 Reformate yield and lower coke formation is having better selectivity Fractional Conversion is defined as say XA = (NA0 NA) / NA0 where XA is the conversion rate of a reactant A, NA0 is the initial concentration of A & NA is the final concentration of A after the reaction takes place1 CHEMICAL REACTIONS r i= ir (4) r i i = r= r A A = r B B = r C C (5) Remember that the stoichiometric coe cients for reactants are negative, while those of products are positive For systems of multiple chemical reactions the rates can be added to obtain the generation of component ifor the whole network of reactions As an example, take the



Al Imam Mohammad Ibn Saud Islamic University A Sta Chegg Com



Fractional Conversion An Overview Sciencedirect Topics
A chemical reaction 8 The existence of a substance that enters in one inlet stream and leaves in one outlet stream with known compositions and it passes unchanged through the process unit (inert for chemical reaction) is greatly simplified material balance calculations This substance is termed as (tie component) It is important to searchUsually, you have to calculate the theoretical yield based on the balanced equation In this equation, the reactant and the product have a 11 mole ratio, so if you know the amount of reactant, you know the theoretical yield is the same value in moles (not grams!)You take the number of grams of reactant you have, convert it to moles, and then use this number of moles to find out how manyYield of S when fractional conversion of A is 05?



Limiting Reactant And Reaction Yields Article Khan Academy


Q Tbn And9gcsgoqphmfu9xy1k3qgcynhdl Mpu5byvptmwuu3zrhifasci5mw Usqp Cau
Multiple choice question Examine how fractional conversion relates between species in a reactionAnnotations must be turned on to properly use this interactive screencast Made by faculty at the University of Colorado Boulder, Department of Chemical & Biological EngineeringBecause 28 is 1/th of 56, so theoretically you can get 1/th of g of FeS or 44g 2nd the % yield calculation itself % yield = actual amount obtained x 100 / maximum theoretical amount possible % yield = 41 x 100 / 44 = 932% (to 1dp, 3sf) More examples of % yield and atom economy calculations in section 6The following chemical reaction takes place ∑ i = 1 n ν i A i = ∑ j = 1 m μ j B j {\displaystyle \sum _{i=1}^{n}\nu _{i}A_{i}=\sum _{j=1}^{m}\mu _{j}B_{j}} , where ν i {\displaystyle \nu _{i}} and μ j {\displaystyle \mu _{j}} are the stoichiometric coefficients


Notes Introduction To The Descriptive Equations For Chemical Reactors Altamira Instruments


Http Www Velhightech Com Documents Bt6604 Cre Pdf
Chemical Engineering July 1997 74 Shelley, Suzanne "Winds of change Out of thin air" Chemical Engineering June 1991 3642 Summers, D (18, February) Bubble Cap Tray Vapor Turndown Chemical Engineering Essentials for the CPI Professional, 3841Exceed the number of chemical species in the process 5) Write down the equations you will solve Try to write them in an order that will simplify the calculations For example, write equations with only one unknown first, as these can be solved right away and, once solved, will eliminate an unknown from subsequent calculations 6) Solve theChemical Engineering July 1997 74 Shelley, Suzanne "Winds of change Out of thin air" Chemical Engineering June 1991 3642 Summers, D (18, February) Bubble Cap Tray Vapor Turndown Chemical Engineering Essentials for the CPI Professional, 3841


Ceng Tu Edu Iq Ched Images Lectures Chem Lec St1 C3 Basic 1 Pdf



3 1 Chemical Equations Chemistry Libretexts
To use this formula for percent yield, you need to make sure that your actual yield and theoretical yield are in the same units If the actual yield is in grams, then theoretical yield also needsChemical Cyclohexane T oluene Although the yield for the fractional distillation of toluene was not above 80%, it was still separated from the mixture Chemical Engineering ScienceThe absolute yield can be given as the weight in grams or in molar (molar yield) while the fractional yield or relative yield or percentage yield is calculated by dividing the amount of the obtained product in moles by the theoretical yield in moles The percentage yield is obtained by multiplying the fractional yield by 100%


Chemeng Queensu Ca Courses Chee221 Files Module 3a mbs with reaction Pdf


Http Faculty Poly Edu Rlevicky Handout3 Pdf



Guidelines For Performing Lignin First Biorefining Energy Environmental Science Rsc Publishing Doi 10 1039 D0eec



Mass And Energy Balances



Calculating Reaction Yield And Percentage Yield From A Limiting Reactant Science Class Video Study Com


Http Www Velhightech Com Documents Bt6604 Cre Pdf



Solutions And Solution Properties Chapter 1 Handbook Of Industrial Crystallization



Quiz 2 15 Solutions Chemical Reactor Carbon Monoxide


Q Tbn And9gcrynatxciaobbvt Edbzcqmlgnhpv Acw5cqav2pwzm1fudi Ih Usqp Cau


Chapter 6 Summary Notes



Multiple Reactions Yield Selectivity Youtube



Fractional Conversion An Overview Sciencedirect Topics



What Are Luminescence Quantum Yields Horiba


Chapter 6 Summary Notes


Ceng Tu Edu Iq Ched Images Lectures Chem Lec St1 C3 Basic 1 Pdf



Stoichiometric Imbalance



Fractional Conversion An Overview Sciencedirect Topics


Pubs Acs Org Doi Pdf 10 1021 Iea022



Percent Yield Calculator Find Percent Yield With Formula Equation



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Notes Introduction To The Descriptive Equations For Chemical Reactors Altamira Instruments
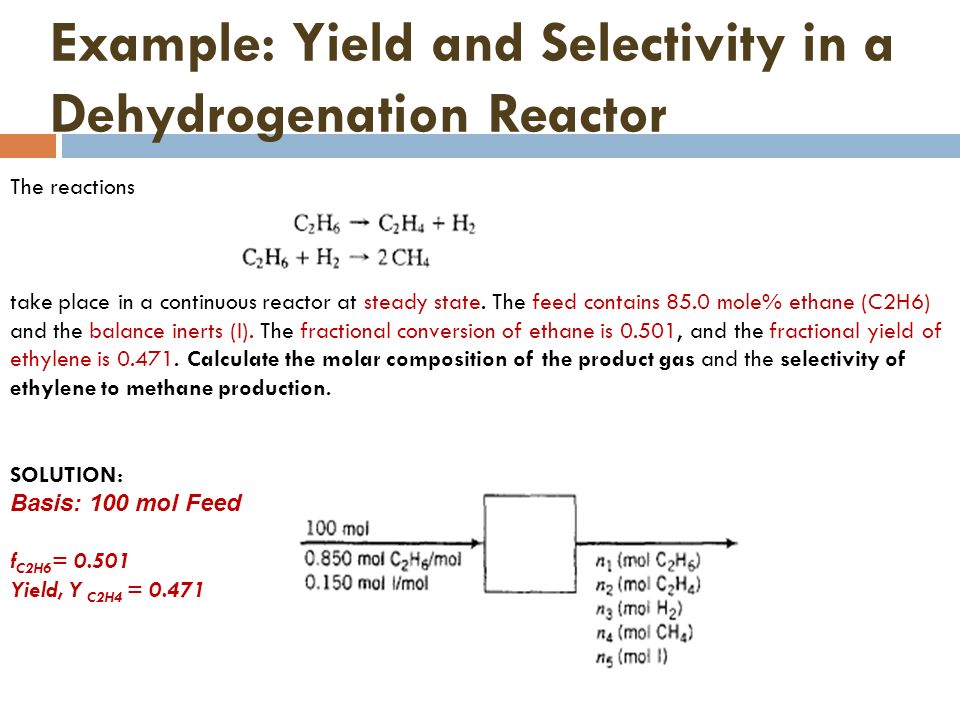


Chapter 3 Material Balance Part Ii Ppt Video Online Download
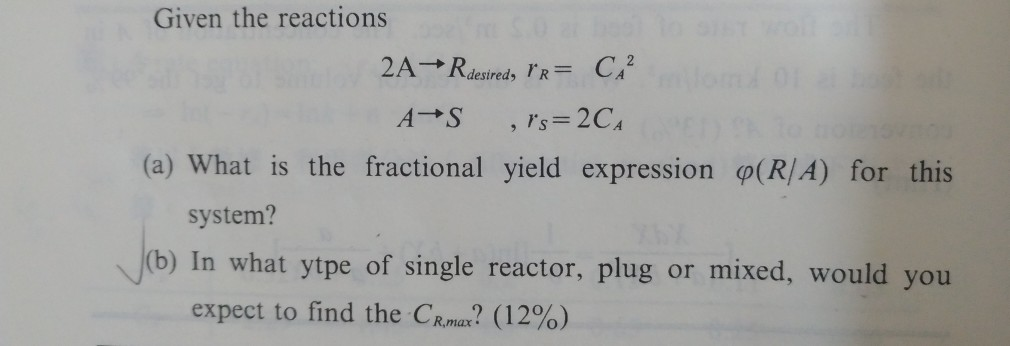


Solved Given The Reactions A What Is The Fractional Yie Chegg Com



Aspen Plus Chemical Engineering Applications Part 1 By Le Xuan Tung Issuu



Fractional Conversion Youtube



C3a Working With Multiple Reactions Yield Selectivity Youtube


Chapter 6 Summary Notes


Elements Of Chemical Reaction Engineering



Mass And Energy Balances



Chapter 3 Material Balance Part Ii Ppt Video Online Download



Chapter 3 Material Balance Part Ii Ppt Video Online Download



Answered Problems 1 4 67 Methane And Oxygen Bartleby
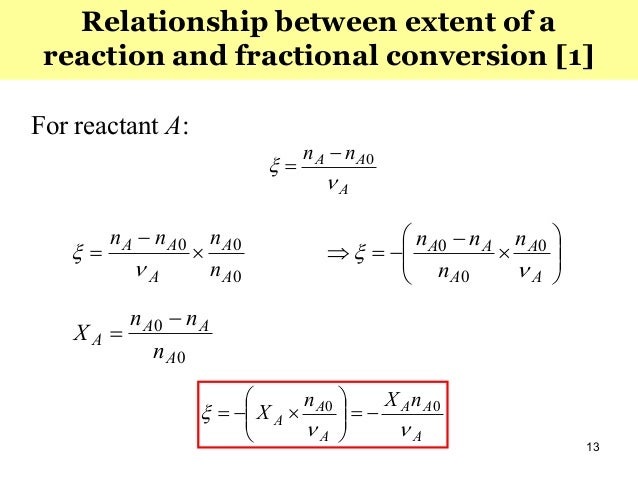


Advanced Chemical Reaction Engineering Part 1 10 Apr 16



Percent Yield Calculator For Chemistry Equations


Http Www Kau Edu Sa Files Files 5877 Che1ch9 Pdf
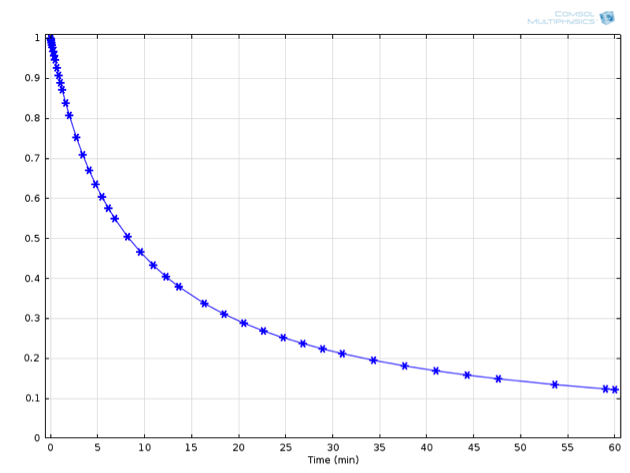


A General Introduction To Chemical Kinetics Arrhenius Law Comsol Blog



Percent Yield Calculator For Chemistry Equations
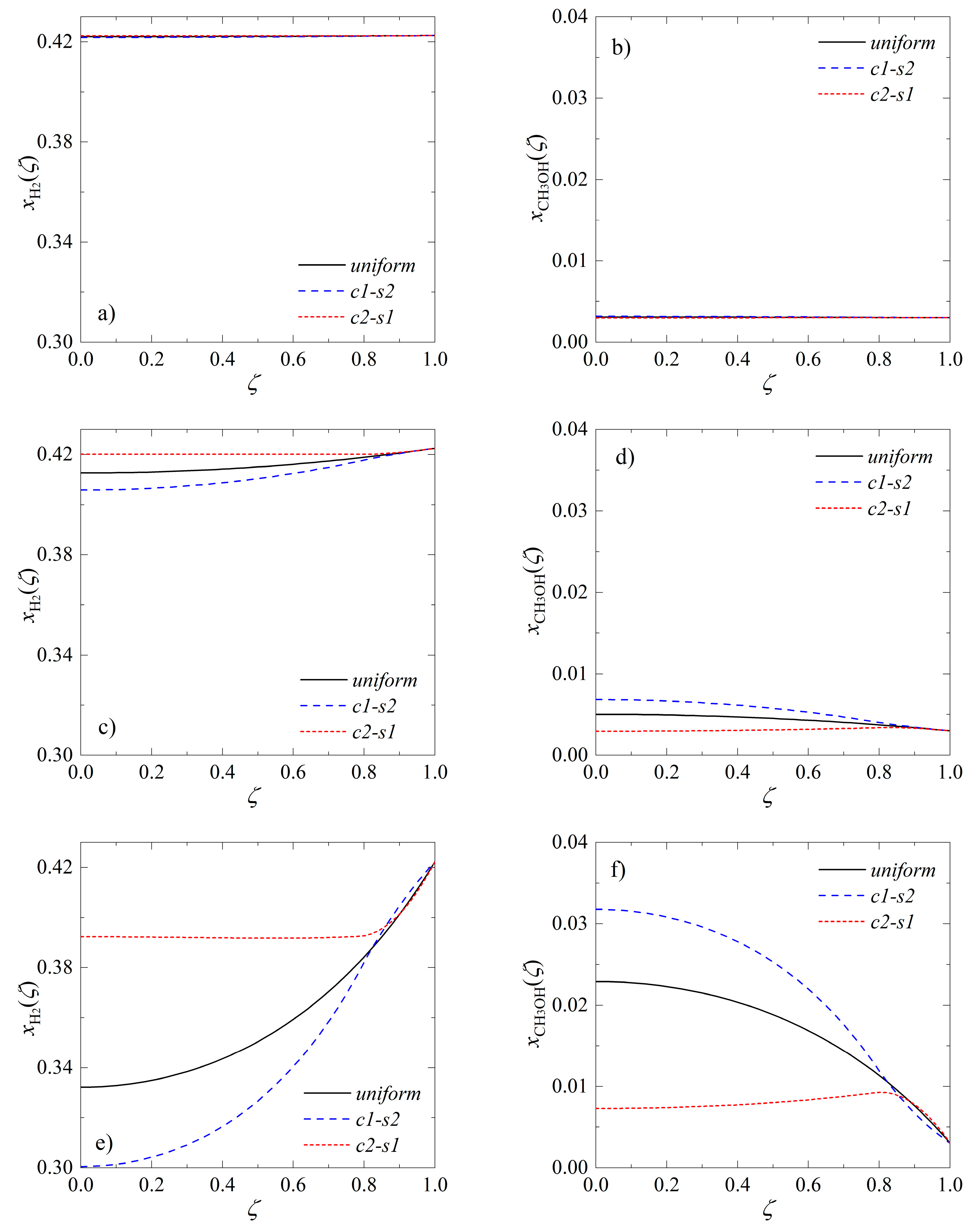


Catalysts Free Full Text Intensification Of Catalytic Processes Through The Pellet Structuring Steady State Properties Of A Bifunctional Catalyst Pellet Applied To Generic Chemical Reactions And The Direct Synthesis Of Dme



How To Calculate Percent Yield Definition Formula Example Chemistry Class Video Study Com


Rmp Lecture Notes



The Effects Of Mixing Reaction Rates And Stoichiometry On Yield For Mixing Sensitive Reactions Part I Model Development



Input Generation Output Consumption Ppt Download


Introduction To Chemical Engineering Processes Vapor Liquid Equilibrium Wikibooks Open Books For An Open World


Chemeng Queensu Ca Courses Chee221 Files Quiz 2 15 solutions Pdf



Fractional Conversion Interactive Youtube



Co Po Chemical Process Principles Studocu


Determination Of Optimal Catalyst Concentration For Maximum Biodiesel Yield From Tigernut Cyperus Esculentus Oil



Solved A Revisit Examples 2 1 Through 2 3 How Would Your An Chegg Com



How To Calculate Percent Yield In Chemistry 15 Steps


The Pillars Curriculum For Chemical Engineering



Industrial Crystallization Biwic 19 Chemical Engineering Technology Vol 43 No 6
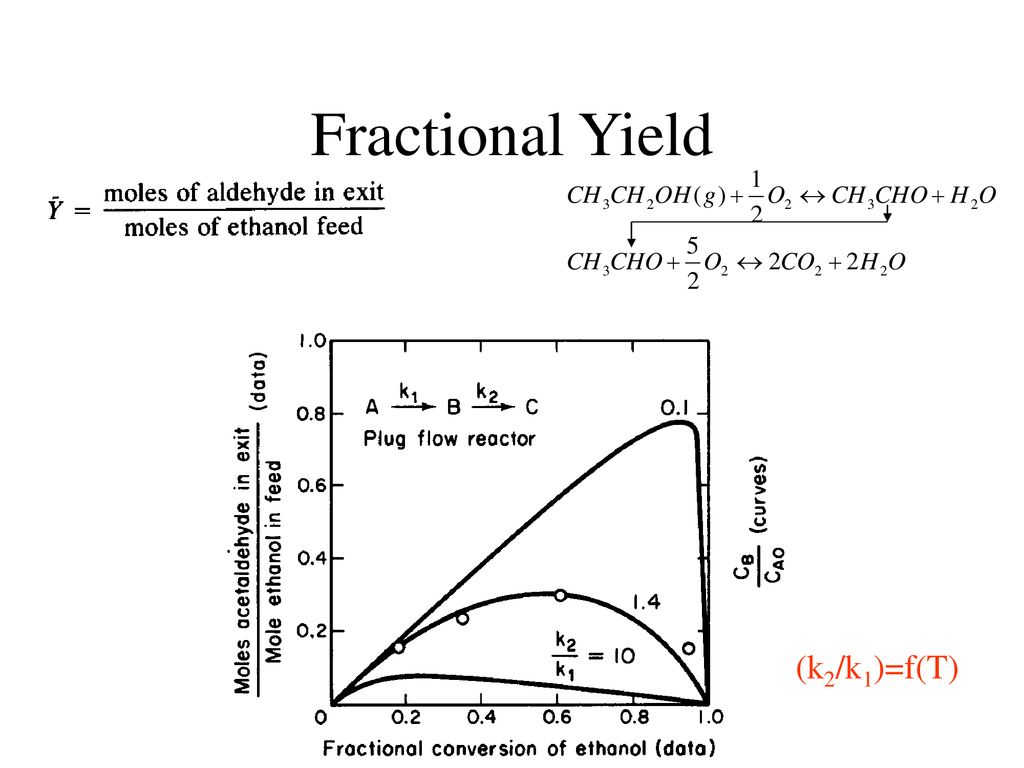


Reactor Design For Selective Product Distribution Ppt Download



Perry S Chemical Engineers Handbook Eighth Edition Mcgraw Hill Education Access Engineering



Gtu Be Question Paper Sem Vi Chemical Reaction Engineering I Summer 19



How To Calculate Percent Yield In Chemistry 15 Steps
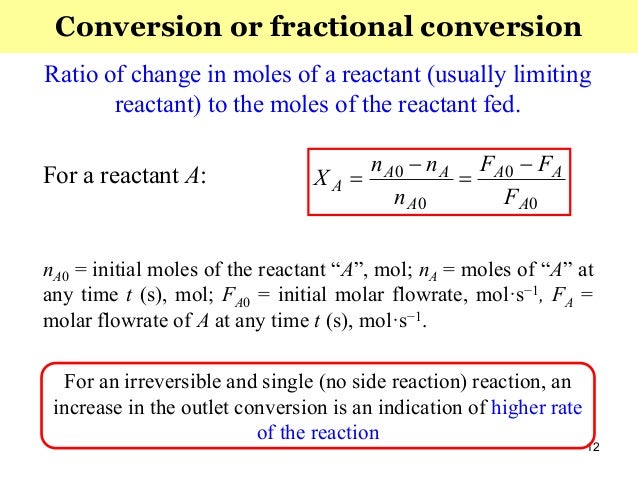


Advanced Chemical Reaction Engineering Part 1 10 Apr 16



Pdf Reactors In Process Engineering


Http Www Just Edu Jo Yahussain Files Reactors Pdf



Fractional Conversion An Overview Sciencedirect Topics



Chemical Engineering Calculation Chemical Engineering Calculations Stoichiometry Studocu



The Effects Of Mixing Reaction Rates And Stoichiometry On Yield For Mixing Sensitive Reactions Part I Model Development



Chemical Equations Protocol


Web Ung Edu Media Chemistry Chapter4 Chapter4 Stoichiometryofchemicalreactions Pdf


Research Aston Ac Uk Files Kinetic Reaction Model For Biomass Pyrolysis Processes In Aspen Plus Pdf


Introduction To Chemical Engineering Processes Vapor Liquid Equilibrium Wikibooks Open Books For An Open World


Ceng Tu Edu Iq Ched Images Lectures Chem Lec St1 C3 Basic 1 Pdf


Cfd Simulation In Chemical Reaction Engineering



Material And Energy Balances Zybooks



Material And Energy Balance



Practical Fundamentals Of Chemical Engineering Eit Engineering Institute Of Technology Eit Engineering Institute Of Technology


Reactor Design And Analysis Introduction To Chemical And Biological Engineering



Stoichiometry Wikipedia
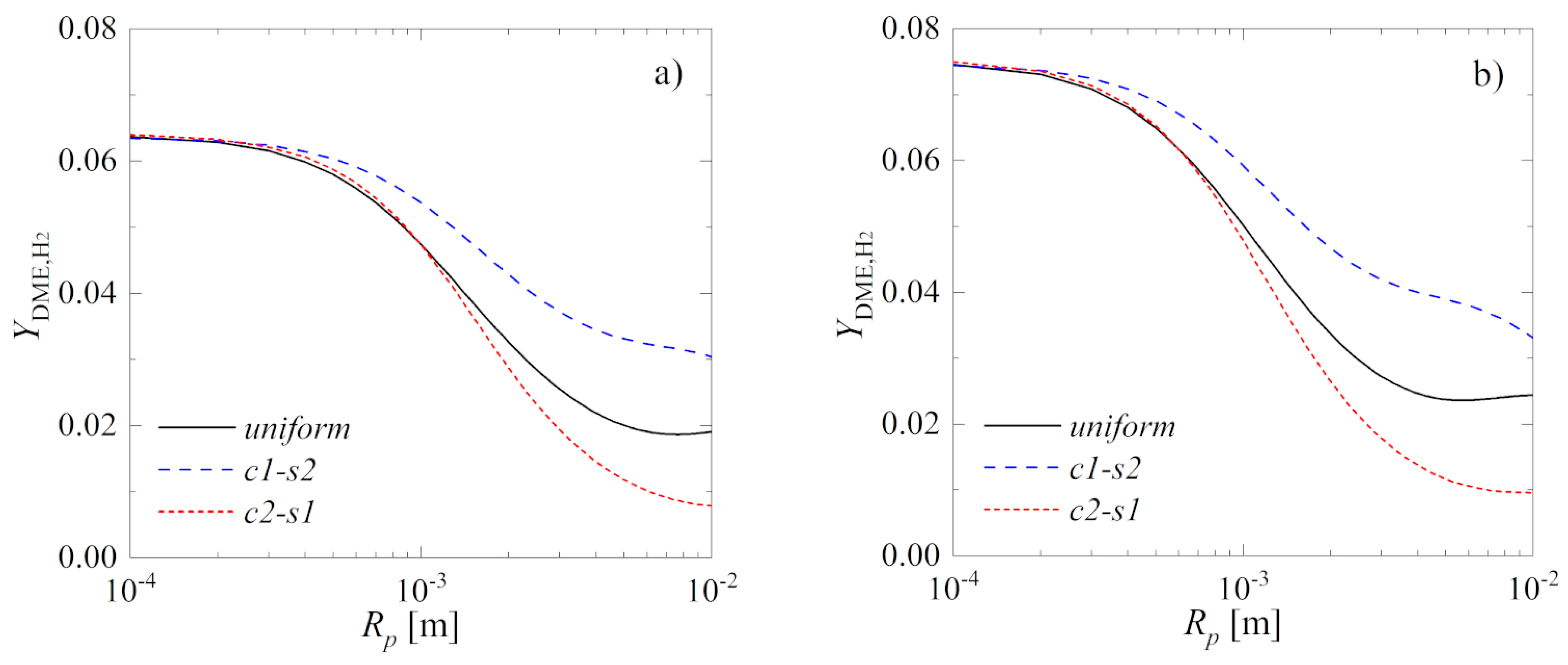


Catalysts Free Full Text Intensification Of Catalytic Processes Through The Pellet Structuring Steady State Properties Of A Bifunctional Catalyst Pellet Applied To Generic Chemical Reactions And The Direct Synthesis Of Dme



Fractional Conversion An Overview Sciencedirect Topics


Notes Introduction To The Descriptive Equations For Chemical Reactors Altamira Instruments



Two Reactions Extent Of Reaction Method Youtube



Chapter 11 Combustion Updated 5 31 10



Chemical Equations Protocol


Http Www Velhightech Com Documents Bt6604 Cre Pdf


Reflux Wikipedia


コメント
コメントを投稿